
Insights+: EMA Marketing Authorization of New Drugs in November 2023
Shots:
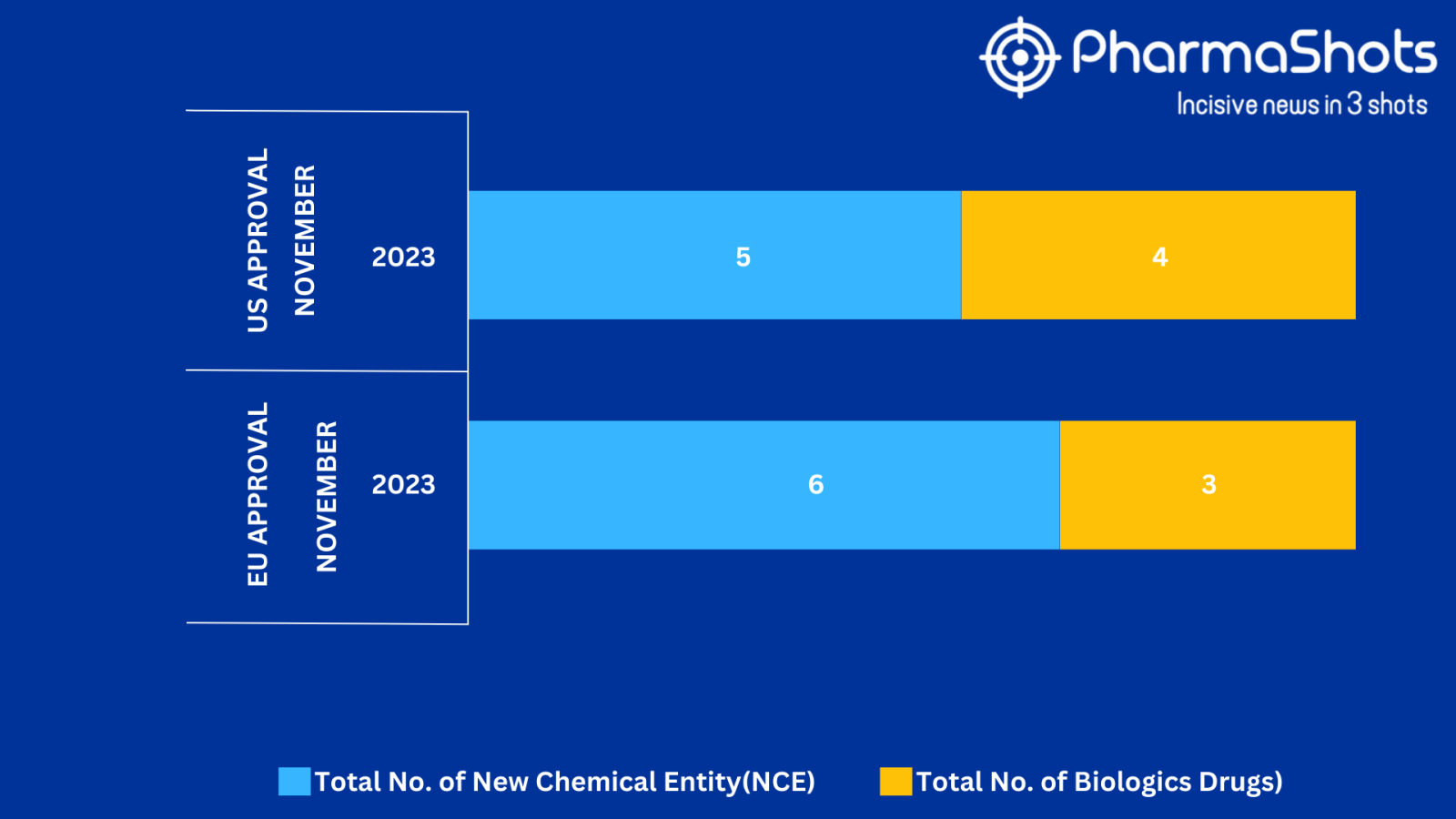
- The EMA approved 3 BLA while 6 New Chemical Entities with 1 recommendation and 1 acceptance in November 2023, leading to treatments for patients and advances in the healthcare industry
- In November 2023, the major highlighted drugs were Vyjuvek to Treat Dystrophic Epidermolysis Bullosa (DEB) and Vyvgart with Halozyme’s Enhanze for the treatment of Generalized Myasthenia Gravis
- PharmaShots has compiled a list of a total of 9 new drugs approved by the EMA in November 2023
Vyjuvek NCE
Active ingredient: Beremagene geperpavec Date: Nov 27, 2023
Company: Krystal Biotech Disease: Dystrophic Epidermolysis Bullosa (DEB)
- The EMA has validated the MAA of Vyjuvek for the treatment of DEB & is now under CHMP review. A CHMP opinion is anticipated in the H2’24
- In Sep 2023, the company also received a positive opinion from the EMA Pediatric Committee on the Pediatric Investigation Plan for Vyjuvek to treat DEB that will make the company eligible for up to an additional 2yrs. of marketing exclusivity in the EU, on top of the 10yrs.
- Previously, Vyjuvek received ODD & PRIME eligibility from the EMA
Bayer Reports EMA's Recommendation for the Approval of Aflibercept to Treat nAMD & Macular Edema
Aflibercept
Active ingredient: Aflibercept Date: Nov 14, 2023
Company: Bayer Disease: nAMD & macular edema
- The company’s aflibercept (8mg, Q4W) has received positive CHMP opinion after 3 initial monthly doses with extension up to Q5W dosing in patients with stable visual outcomes for the treatment of neovascular (wet) age-related macular degeneration (nAMD) and diabetic macular edema (DME). The EC’s decision is expected by YE’23
- The positive opinion was based on P-III (PULSAR) trial in nAMD and P-II/III (PHOTON) trial in DME evaluating efficacy and safety of aflibercept. Both studies met their 1EP of non-inferior BCVA changes at 12 and 16wks. dosing regimen vs aflibercept (2mg, Q8W) dosing at wk. 48
- The EC’s decision is anticipated in the coming months. Bayer has applied for the brand name of aflibercept 8 mg to be ‘Eylea 114.3 mg/ml solution for injection’ (Eylea™ 8mg).
Valneva Reports EMA Acceptance of VLA1553 for Chikungunya
Chikungunya Vaccine (VLA1553)
Active ingredient: NA Date: Nov 27, 2023
Company: Valneva Disease: Chikungunya
- The EMA has formally validated the MAA for VLA1553, confirming inclusion of essential regulatory elements followed by the accelerated assessment granted by the CHMP last month
- In early Dec, VLA1553 received approval from the US FDA under the brand name IXCHIQ for the prevention of disease caused by the chikungunya virus (CHIKV) in 18yrs. and older patients who are at greater risk to CHIKV
- Additionally, in mid-Nov, Valneva revealed P-III immunogenicity results for VLA1553 in adolescents, supporting a potential label extension. The trial aims to facilitate vaccine licensure in Brazil, marking a potential first approval for use in endemic populations
Kaftrio + Ivacaftor
Active ingredient: ivacaftor, tezacaftor & elexacaftor Date: Nov 23, 2023
Company: Vertex Pharmaceutical Disease: Cystic Fibrosis
- Vertex Pharmaceutical receives EC’s approval to expand the label of Kaftrio + Ivacaftor for treating children aged 2 to 5 with cystic fibrosis (CF) who have at least one F508del mutation in the CFTR gene
- The real-world data from the trial demonstrated the clinical benefit of Kaftrio in CF among young children across Europe, extended Kaftrio indication will soon be available to patients in Austria, Denmark, Ireland, Norway, Latvia, & Sweden
- Kaftrio in combination with ivacaftor is an oral medicine aimed at enhancing both the quantity and functionality of the CFTR protein on the cell surface
Senvelgo
Active ingredient: Velagliflozin Date: Nov 23, 2023
Company: Boehringer Ingelheim Disease: Hypoglycemia
- The EC approved Senvelgo (velagliflozin oral solution), a prescription medication to improve glycemic control in cats with diabetes mellitus
- Senvelgo reduces blood glucose levels & minimizes the risk of clinical hypoglycemia episodes, improving the clinical symptoms of diabetes in cats as soon as one week after starting treatment. The oral liquid solution can be given QD to cats directly into their mouths or with small amounts of food
- Senvelgo oral solution, a highly selective inhibitor of the sodium-glucose co-transporter 2 (SGLT2) will be available in Europe, has already received approval in Switzerland, Great Britain, the U.S & seeking regulatory approval in the ROW
Yorvipath
Active ingredient: Palopegteriparatide Date: Nov 20, 2023
Company: Ascendis Pharma Disease: Chronic Hypoparathyroidism
- The approval was granted following the CHMP’s positive opinion for Yorvipath adopted earlier on Sep’23. The CHMP positive opinion and recommendation for approval was granted to Yorvipath by the name TransCon PTH (palopegteriparatide) as a parathyroid hormone (PTH) replacement therapy for the treatment of adults with chronic hypoparathyroidism
- The CHMP positive opinion was granted based on the data from the P-III (PaTHway) & P-II (PaTH Forward) clinical trials evaluating TransCon PTH in patients with hypoparathyroidism
- TransCon PTH, to be marketed in the EU as Yorvipath, is a prodrug of parathyroid hormone (PTH 1-34) indicated to be administered QD. Moreover, the company plans to launch Yorvipath initially in Germany by Jan’24
Ebglyss
Active Ingredient: lebrikizumab Date: Nov 17, 2023
Company: Almirall Disease: Moderate-to-severe Atopic Dermatitis
- The approval was based on 3 P-III clinical trials incl. (ADvocate 1) & (ADvocate 2) evaluating lebrikizumab as monotx. & (Adhere) evaluating lebrikizumab + TCS in adult & adolescent patients (n=1300) with moderate-to-severe atopic dermatitis
- The results showed that disease extent & severity reduced by 75% (EASI-75) in 6/10 & 7/10 patients receiving monotx. & combination therapy at 16wks. & ~80% of the 16wks. responder depicted sustained skin clearance, itch relief & reduced disease severity
- Almirall has the license to develop & commercialize lebrikizumab for dermatology indications incl. AD in Europe whereas Eli Lilly holds the exclusive development & commercialization rights for lebrikizumab in the US & ROW
Obinutuzumab
Active Ingredient: obinutuzumab Date: Nov 17, 2023
Company: BeiGene Disease: Follicular Lymphoma
- The approval was based on the data from the P-II (ROSEWOOD) clinical trial evaluating Brukinsa + Obinutuzumab vs Obinutuzumab alone in patients (n=217) with r/r follicular lymphoma
- The results from the study depicted an ORR of 69% vs 45.8% with a median follow-up of ~20mos. whereas DoR was found to be 69.3% in patients being treated with the combination therapy & mPFS was 28.0mos. vs 10.4mos.
- Brukinsa, a BTK inhibitor, has been approved as a monotx. in the EU & the company has planned submissions for the regulatory review of Brukinsa by the US FDA & NMPA. Moreover, the applications for Brukinsa are under review by the regulatory authorities of Canada, Switzerland & the UK
Vyvgart
Active Ingredient: efgartigimod alfa + rHuPH20 Date: Nov 16, 2023
Company: argenx Disease: Myasthenia Gravis
- The approval was granted based on the results from the P-III (ADAPT-SC) clinical trial evaluating the PD effect of Vyvgart as an SC (1000mg efgartigimod-PH20) vs IV (10mg/kg) dose in patients (n=110) with gMG across North America, EU & Japan
- The study met its 1EP depicting a mean total IgG reduction of 66.4% from baseline vs 62.2%. The 2EPs of the trial were also met with 69.1% & 65.5% of SC-treated patients were responders on MG-ADL & QMG score
- argenx’s Vyvgart is designed with the combination of efgartigimod alfa + rHuPH20 & is co-formulated with Halozyme’s Enhanze drug delivery technology. Earlier, Vyvgart received the CHMP’s positive recommendation & was approved by the US FDA on Jun’23 for gMG

Shivani is a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She covers news related to Product approvals, clinical trial results, and updates. She can be contacted at connect@pharmashots.com.













